Most of the locally defined terms in this glossary are ones that have significant and particular application to human life expansion. Many of them have one or more technical meanings within the sciences related to life expansion that are different from that of the common usage, or even within other sciences. In such cases, we have chosen to include only the meaning(s) relevant to the subject(s) covered within MoreLife. In constructing our definitions we wish to acknowledge the value of these references.
In order to prevent unnecessary duplication, we have selected several excellent sources for external definitions. However, since our usage of some terms is quite specialized and we wish to emphasize certain parts of their meanings, we often locally define terms from these dictionaries within this glossary. In addition, because many anatomical parts and various specific diseases may be of interest to the reader, links to well presented glossary information on other websites have been provided.
| paracrine |
Describing or relating to a regulatory cell that secrets an agonist into the intercellular spaces in which it diffuses to a target cell other than that which produces it; describing or relating to such an agonist. |
pathological
pathology |
Of or related to pathology - the science which deals with the causes and nature of diseases and the effects of these on the structure and functioning of the organism. The sum of such effects and changes.
A pathological cell or tissue is therefore a diseased or abnormal cell or tissue. |
| peer-review (scientific studies) |
The critical scrutiny of the methods and conclusions of the report of an experiment by independent scientists who are knowledgeable in the area of investigation under study. A report of an experiment must undergo such peer-review, which may require modification of the study or even complete rejection of the study report, before publication is allowed in a peer-reviewed journal, and, in this manner, the report of the experiment is transformed into a scientific study.
Contrast this with a report of an experimental investigation at a conference. In that case, the report is presented in summary before some peers of the investigator, but no peers have the chance, nor are designated responsibility, for its detailed critical scrutiny. Thus, even though the report may be published in the conference proceedings, it has not been through the same critical scrutiny as that published in a peer-reviewed journal, and should not be given the same evidential weight. |
| peptide |
Any compound containing two or more amino-acid residues joined by amide bond(s) formed from the carboxyl group of one amino acid (residue) and the amino group of the next one by dehydration (the removal of a water molecule composed of the OH from the carboxyl group and an H from the amine group). The term peptide usually applies to compounds in which the residue linkage is formed between the carboxyl carbon atom adjacent to the 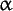 -carbon (see structure formula) of one -carbon (see structure formula) of one 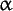 -amino acid (residue) and the nitrogen atom connected to the alpha carbon of another, but it includes compounds in which other carboxyl carbons or amine nitrogens are linked. Peptide bond structure -amino acid (residue) and the nitrogen atom connected to the alpha carbon of another, but it includes compounds in which other carboxyl carbons or amine nitrogens are linked. Peptide bond structure |
| peroxide |
Any compound of general formula R-O-O-R', where R and R' may be different or identical compounds. |
phagocyte
phagocytosis
endocytosis |
Any cell that characteristically engulfs particles from its surroundings into its cytoplasm. Phagocytosis is a type of endocytosis (uptake of external materials by cells) whereby certain cells - phagocytes - can engulf external particulate material by extension and fusion of pseudopods around each particle. The particles are initially contained within phagocytic vacuoles which then fuse with primary lysosomes to effect digestion of the particles. Phagocytosis is employed by certain cells of the immune system, notably macrophages and neutrophils, to engulf and destroy target cells, tissue debris, and other particles. |
pharmacological
(dose, effect) |
A concentration or dose of a naturally occuring agonist having an unphysiological effect.
The effect or activity of such a dose. Compare physiological, pathological, normal. |
phenol
phenolic |
Any compound containing one of more hydroxyl groups directly attached to an aromatic carbocycle. Phenols are weakly acidic. |
| phenyl |
The aromatic carbocyclic group, C6H5 - derived from benzene. |
| phosphoric |
The general name for a residue of [ortho]phosphoric acid - ie. tetraoxophophoric acid, PO(OH)3 - whether singly, doubly, or triply attached to other groups and whether or not any residual hydroxyl groups are deprotonated (proton removed in the process). |
physiology
physiological |
The science dealing with the functioning of the cells, tissues, organs, and organisms and with the chemical and physical phenomena concerned.
physiological: of or pertaining to physiology. |
| physiological (dose, effect) |
A normal (dose, effect). Not pathological or pharmacological. |
pi bond
pi electron
delocalized |
A pi or 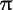 bond is the type of covalent bond formed between atoms by electrons occupying p orbitals. In particular, when one carbon is double bonded to another, one of the bonds is always a pi bond and the other a sigma bond. The electrons in the pi bond are outside the plane of the sigma bonds between the atoms of the compound and can move about more freely. When a carbon chain or ring of a compound has sufficient symmetry with respect to alternating single and double bonds, the pi electrons roam freely between all carbons and the electrons and bonds are thus called delocalized. In particular, one pair of bond electrons for each double bond in benzene rings and conjugated chains are delocalized. Delocalized pi electrons are one type of electron arrangement which cause a molecule to exhibit resonance. bond is the type of covalent bond formed between atoms by electrons occupying p orbitals. In particular, when one carbon is double bonded to another, one of the bonds is always a pi bond and the other a sigma bond. The electrons in the pi bond are outside the plane of the sigma bonds between the atoms of the compound and can move about more freely. When a carbon chain or ring of a compound has sufficient symmetry with respect to alternating single and double bonds, the pi electrons roam freely between all carbons and the electrons and bonds are thus called delocalized. In particular, one pair of bond electrons for each double bond in benzene rings and conjugated chains are delocalized. Delocalized pi electrons are one type of electron arrangement which cause a molecule to exhibit resonance. |
polymorphic cell
poly+
morphic+ |
A cell which can take several forms or styles. From poly - a combining form meaning "many", and morphic - a combining form meaning shape or structure. |
polyunsaturated
omega-3
omega-6 |
Pertaining to a fatty acid which contains two or more double bonds between two of its carbons. However, in common usage the term is narrowed to mean only those straight chain fatty acids in which a pair of double bonded carbons are separated by two single bonded carbons. As with monounsaturated fatty acids, polyunsaturates contained in foods are classified by the distance from the omega carbon of their closest double bond. The reason for this it because the human body does not produce any enzyme which can insert a double bond nearer than 9 carbons from the terminal carbon. In practice this means that certain categories of polyunsaturated fatty acids cannot be generated by the human body nor can they be interconverted. Because of this limitation, two classes of fatty acids (omega-3 and omega-6 - those having double bonds between the 3,4 and 6,7 carbons from the terminal omega end, respectively) have been found to be essential nutrients in the human diet. Polyunsaturated fats occur naturally mainly in the cis form, but trans forms can be generated by bacteria in the rumen and may be contained in ruminant products. Trans forms can also be generated by the hydrogenation of polyunsaturated fats. |
| protein |
Any of a large group of organic compounds found as major macromolecular constituents of living organisms. All enzymes are proteins, although catalytic activity is shown by some nucleic-acid molecules (see ribozyme). A protein is a linear polymer of amino acids linked by peptide bonds in a specific sequence. In the biosynthesis of the polypeptide chain, any of 20 different amino acids may be incorporated, according to the genetic instructions of the cell. The amino-acid residues may be modified subsequently so that the chains may contain a much wider variety of residues, amounting to nearly 200. The modifications may involve the covalent attachment of various groups such as carbohydrates and phosphate; these are the simple proteins. Other substances may be more loosely associated with the polypeptide chains, such as heme or lipid, giving rise to the conjugated proteins. Nutritional aspects of protein metabolism include the fact that higher animals cannot synthesize certain of the amino acids required for protein synthesis; such amino acids are referred to as essential amino acids. Plants and most microorganisms can synthesize all of the amino acids required for protein synthesis and many others. The nutritional value of different proteins depends on their composition. Some proteins are of poor nutritional value due to total or relative deficiency of essential amino acids, eg. gliadin (a major protein in grains), which lacks lysine, and zein (the major protein in corn), which is relatively deficient in lysine and tryptophan. |
pyran ring
pyranoid |
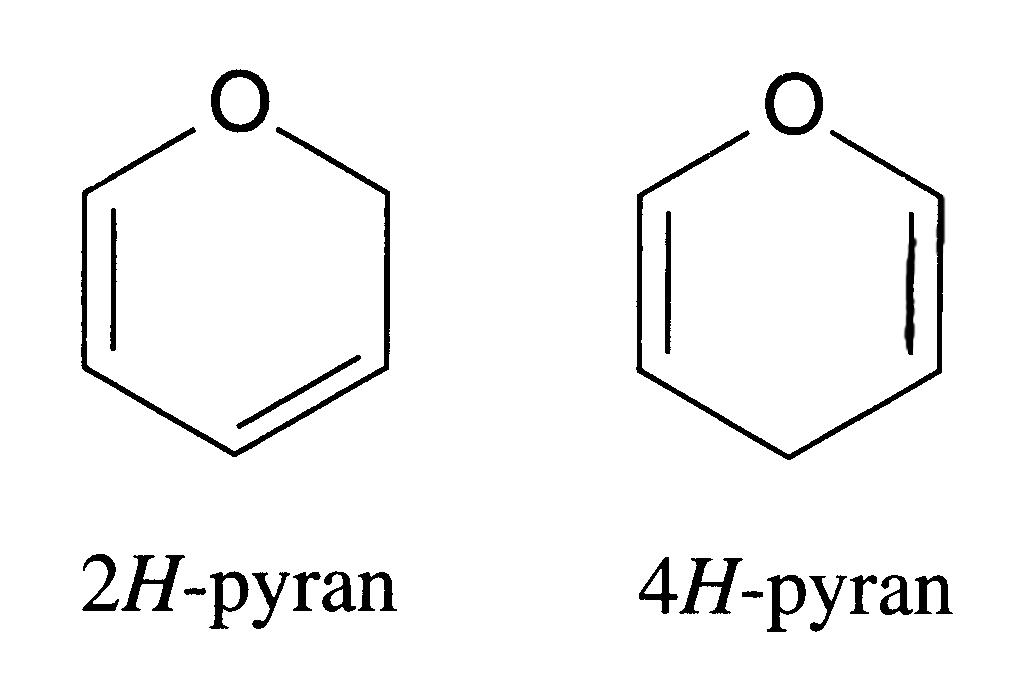 One of two forms of hexagonal heterocyclic rings containing one oxygen and 5 carbons, containing two double bonds. One of two forms of hexagonal heterocyclic rings containing one oxygen and 5 carbons, containing two double bonds.
Pyranoid, pyran-like, i.e. describing a structure that consists of or contains a pyran ring. |
| R (group) |
An unspecified univalent group in a formula of an organic compound. The group must be attached by means of carbon and derived from aliphatic, carbocyclic, or heterocyclic compound. Up to three such groups, when different, may be designated R, R', and R", or R1, R2, and R3. |
radical
free radical
radical pair |
(in chemistry) Any molecular entity, charged or uncharged, that possesses an unpaired electron (but normally excluding any paramagnetic metal ion); often formed by homolysis of a covalent bond. Radical character is indicted in a formula by a centered dot symbolizing the unpaired electron; eg.HOO•, •CH3. The term free radical is now preferably restricted to any radical that does not form part of a radical pair. (Radical pair: any two radicals in close proximity in liquid solution, with a solvent cage. They may have been formed simultaneously, eg. by homolysis, or have come together by diffusion. While together, correlation of their unpaired electron spins occurs and manifests itself as a distortion of nuclear magnetic resonance (NMR) spectra.) |
| reaction |
(In chemistry) any process in which molecules interact with one another, leading to chemical or physical (pertaining to matter and energy) change. |
| reaction intermediate |
A transient chemical species, with a lifetime appreciably longer than a molecular vibration time, formed directly or indirectly from the reactants of a chemical (often enzyme reaction, and that further reacts (directly or indirectly) to give the reaction products. |
| reducing agent |
Any substance acting as a reductant, that is, donates an electron in an oxidation-reduction reaction. The species that undergoes oxidation in such a reaction. |
reduction
reduce |
The chemical process by which oxygen is withdrawn from, hydrogen is added to, or (more generally) an electron is added to (with or without addition of a proton) a molecular entity. Compare oxidation.
Reduce: to cause or undergo reduction. |
| resonance |
A phenomenon shown by any molecular entity to which more than one contributing structure can be assigned, when these structures have similar energy and differ only in the distribution of the valence electrons; the entity then oscillates between the different structures and adopts, in effect, an intermediate state. |
| restitution |
The transfer of value to a victim by the person or persons who have violated him up to the full amount and type of value necessary to restore the integrated life happiness of the victim to what it would have become if the violation had not occurred, including all costs of obtaining the restitution. The amount and kind of restitution necessary for full restoration of the victim's integrated life happiness is largely determined by the victim himself because he is the only one who can know his own values well enough to determine the amount of reduction of his integrated life happiness which resulted from the violation. |
| risk factor |
A factor that causes a person or group of people to be particularly vulnerable to an unwanted, unpleasant, or unhealthful event. Often wrongly used to describe a factor which has been determined by an epidemiological study to be correlated with such events. In addition, something which may be a risk factor for one person, or one situation of life parameters is not necessarily a risk factor for a different person or one with different life parameters. For example a suppressed immune system is a risk factor for the incidence and severity of infection, but merely taking an agent which suppresses some part of the immune system, while at the same time taking other agents which enhance other parts of the immune system will not necessarily be a risk factor for such infection. |
R/S System
Cahn-Ingold-Prelog System
CIP System
R/S naming convention |
An absolute nomenclature scheme for molecules formulated in 1956 by Robert Cahn, Christopher Ingold, and Vladimir Prelog. In this CIP system, the four groups surrounding a chiral center are ranked according to a specific priority scheme; Atoms of higher atomic number bonded to a chiral center are ranked above those of lower atomic number.
The order of priority of some common functional groups is
SH > OH > NH2 > COOH > CHO > CH2OH > C6H5 > CH3 > 2H > 1H
The prioritized groups are assigned the letters W, X, Y, Z such that their order of priority rating is W > X > Y > Z. To establish the configuration of the chiral center, it is viewed from the asymmetric center towards the Z group (lowest priority). If the order of the groups W -> X -> Y as seen from this direction is clockwise, then the configuration of the asymmetric center is designated (R) (Latin: rectus, right). If the order of W -> X -> Y is counter clockwise, the asymmetric center is designated (S) (Latin: sinistrus, left). L-Glyceraldehyde is therefore designated (S)-glyceraldehyde and similarly, L-alanine is (S)-alanine. In fact, all the L-amino acids from proteins are (S)-amino acids,with the exception of L-cysteine, which is (R)-cysteine.
For more on R/S Naming convention , Chime plug-in needed for full visualization.
See also, older Fischer scheme D/L convention, awkward and sometimes more ambiguous. |
 One of two forms of hexagonal heterocyclic rings containing one oxygen and 5 carbons, containing two double bonds.
One of two forms of hexagonal heterocyclic rings containing one oxygen and 5 carbons, containing two double bonds.


